

| Hustota je pro určení
minerálů velmi důleitá. U chemicky málo proměnlivých minerálů je toti
při dané teplotě a tlaku vdy stejná a její přesné stanovení někdy stačí
k přímé identifikaci. Je závislá na chemickém sloení nerostu, na jeho
atomové hmotnosti a na velikosti a stavbě molekul, u krystalických
látek pak na krystalové struktuře. U krystalové formy tée sloučeniny
je větí ne u formy amorfní. Při práci v terénu odhadne zkuený sběratel přiblinou hustotu pouhým odváením v ruce. Hovoříme přitom o minerálech lehkých s hustotou 1-2 (některé bitumeny), středně těkých s hustotou 2-4 (sem patří větina nerostů, např. sádrovec a křemen), těkých s hustotou 4-6 (sfalerit, baryt) a velmi těkých s hustotou nad 6 (galenit, kasiterit). Největí hustotu mají ryzí kovy, například zlato 15-16 a platina 14-20. Pro rychlé určení hustoty doma je vhodná a v podstatě dostačující metoda suspenzační. Určujeme zde relativní hustotu minerálů, a to tak, e nezvětralý úlomek minerálu (asi 1 g) ponoříme do skleněné nádobky s tekutinou o známé hustotě. Klesne-li minerál ke dnu, je jeho hustota větí ne hustota tekutiny, plave-li na hladině nebo po ponoření opět vyplave, je jeho hustota mení. Vznáí-li se, odpovídá hustota nerostu hustotě kapaliny. Pro přesnějí měření příslunou tekutinu s nerostem ředíme nebo zahuujeme tak dlouho, a se minerál vznáí (suspenzuje). Pomocí hustoměru nebo pyknometru pak zjistíme hustotu kapaliny a tím i hustotu minerálu. Jako tekutiny můeme pouít např. bromoform (CHBr3) s nejvyí hustotou 2,904 (zřeďuje se éterem nebo benzolem), acetylentetrabromid (C2H2Br4) s hustotou 3,0 (zřeďuje se benzolem), metylenjodid (CH2J2), který má hustotu 3,33 (zřeďuje se alkoholem, éterem, benzolem). Pozor-tyto tekutiny jsou větinou zdraví kodlivé. Nevýhodou této metody je, e neznáme vhodné tekutiny o vyích hustotách. Pro přesné a rychlé stanovení hustoty je vhodná metoda dvojího váení, a to na vzduchu a ve vodě. Je výhodná zejména u neporézních vzorků. K měření hustoty touto metodou můeme pouít upravené laboratorní váhy tak, e na háček nad misku jednoho vahadla zavěsíme na hedvábnou nit daný minerál, který zváíme nejdřív na vzduchu. Pro zváení minerálu ve vodě postavíme na můstek pod upevněný minerál kádinku s destilovanou vodou, její hladina má být tak vysoko, aby byl minerál zcela ponořený. U minerálů rozpustných ve vodě pouijeme jako kapalinu olej, alkohol aj. Po zváení provedeme výpočet hustoty podle vztahu: 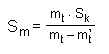
Pro přesné stanovení hustoty velmi malých úlomků minerálů je mono pouít metodu pyknometrickou. Vzorek vloíme do skleněné nádoby (pyknometru) se známým objemem. Po zaplnění měrnou kapalinou a zváení se stanoví objem vzorku. Metoda je náročná na rychlost provedení a tím i na zručnost. Pro tuto metodu platí vztah: 
Hustota nerostů můe značně kolísat navětráním a poruením vzorků. Rovně izomorfní příměsi jiných minerálů a uzavřeniny plynů a tekutin mohou značně ovlivnit výsledek měření. Z těchto důvodů je třeba kadý vzorek před vlastním měřením podrobit zevrubné prohlídce pod lupou nebo binokulárním mikroskopem. |
Another physical
property of minerals, used in their identification, is specific gravity.
In minerals of constant chemical composition, it is always the same at
fixed temperatures and pressures, its exact determination frequently
helps in their identification. It depends upon the chemical composition
of the mineral, on its atomic weight and the molecular size and
arrangement; in crystalline substances it depends upon their crystal
structure. Minerals, in single crystal form, have higher specific
gravities than their polycrystalline equivalents. An experienced collector can estimate the approximate specific gravity by simply holding the mineral and guessing its weight. There are light minerals of a specific gravity 1-2 (some bitumens), medium-heavy minerals of a specific gravity 2-4 (gypsum and quartz), heavy minerals of a specific gravity 4-6 (sphalerite and barytes), and very heavy minerals of a specific gravity of more than 6 (galena and cassiterite). Native metals, such as gold (15-16) and platinum (14-20) have the highest specific gravities. A quick determination of specific gravity may be obtained by the suspension method. The relative specific gravity of minerals is determined when a piece of unweathered mineral (about 1 gm) is submerged in a liquid of known density. If the mineral sinks to the bottom, its specific gravity is higher than that of the liquid; if it floats, its specific gravity is lower. For a more accurate measurement, the liquid is either diluted or concentrated until it achieves the density at which the mineral just floats. Then, it is easy to determine the specific gravity of the liquid by means of a densimeter (ie hydrometer) or pycnometer. A suitable liquid is bromoform CHBr3 with a density of 2.904 (diluted by ether or benzol), acetylene tetrabromide C2H2Br4 of a density of 3 (diluted by benzol), methylene iodide CH2I2 of a density of 3.33 (diluted in alcohol, ether or benzol). The only disadvantage of this method is that liquids of higher density are not readily available, or easy to handle and most of them are harmful. For a quick and exact determination of the specific gravity of a sample, it can be weighed twice, in air and then in water. This method can be applied best to non-porous minerals. A laboratory balance specially adapted for this purpose can be used. On a small hook above one of the scales is hung a small container on a silk thread, which is balanced on the other scale. The mineral sample is placed in the container and weighed in air. To estimate its weight in water, a beakercontaining distilled water is placed under the container so that it is complety immersed. With water-soluble minerals the immersion medium should be oil or alcohol. When the weight of the mineral is known, its specific gravity can be calculated by means of the formula: 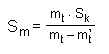
|